Shiga toxin-producing E coli: E coli O157
Introduction
There are various types of E coli that live in the environment and our intestines. Although most live inside our bowel harmlessly and even be beneficial, some E coli may cause infection, like UTI, bacteraemia, gastroenteritis etc.
The E coli causing gastroenteritis could be divided into six phenotypes –
- Enterohaemorrhagic E. coli (EHEC) [also called Shiga toxin-producing E. coli (STEC), or Verocytotoxin-producing E. coli (VTEC)],
- Enterotoxigenic E. coli (ETEC),
- Enteroinvasive E. coli (EIEC),
- Enteropathogenic E. coli (EPEC),
- Enteroaggregative E. coli (EAggEC), and
- Diffusely adherent E. coli (DAEC).
Shiga toxin-producing E. coli (STEC) [or EHEC/VTEC]
STEC is known to cause gastroenteritis (often with visible blood) and haemolytic uraemic syndrome (HUS).
The virulence factors are
- Cytotoxins (stx1 and stx2) – inhibit the host cell’s protein synthesis.
- The outer membrane protein intimin (encoded by the eae gene) helps attach and efface the colon cells. These genes are used as targets for the PCR when we try to identify STEC.
- AggR activator (encoded by the aggR gene): helps in attachment and colonisation.

HUSEC
The STEC can be divided into two types. Those that produce Shiga Toxin 2 (stx2) and those that do not (whether they produce stx1 or not).
The STEC strains that frequently cause HUS are called HUSEC strains. They express Stx2 (stx2a, stx2c, or stx2d) and the eae gene, or aggR gene. Some may produce stx1 as well. Most E. coli O157 strains are HUSEC.
Non-O157 STEC
In the UK, the most common STEC serogroup that causes bloody diarrhoea, and HUS, is O157.
Non-O157 were under-ascertained but are now increasingly being recognised as a cause of serious illness/outbreak.
Examples of non-O157 serogroups are – O26, O146, O55, O91, O103, O128, O145, O104, O111, O121, O80 etc. O26 and O55 are the commonest non-O157 strains isolated from HUS cases.
The annualised rate of STEC – 7.6 cases per 100000 population. The ratio of STEC O157 to non-O157 STEC was 1 to 6.2. [3]
STEC infection
Peak incidence in the summer and early autumn. The incubation period depends upon the number of organisms ingested but is usually 3-4 days (can be up to 14 days). The infectious dose is <100 organisms or even <10 organisms for STEC O157.



STEC may cause –
- Gastroenteritis with abdominal pain and fever. 50-80% of cases have bloody diarrhoea. The diarrhoea usually resolves spontaneously within 7-14 days in uncomplicated cases. Although fever could be present in STEC infection, an alternative diagnosis should be considered if the patient presents with a high fever.
- Haemolytic Uraemic Syndrome (HUS) – is a complication that may affect 11% of cases with STEC infection in the 2nd week of illness. Most (85%) cases are under 16 years old. An abnormal coagulation profile appears first, followed by a drop in platelet count and renal impairment.
- Thrombotic thrombocytopaenic purpura (TTP)
- Intussusception
STEC can affect any age, but children <10 years old and older adults are the most vulnerable.
Outbreaks may occur in establishments such as nursing homes and following visits to open farms.
The infection may be acquired from
- Ingesting contaminated food/water – contaminated cooked meat (ground beef, beef burgers), contaminated or unpasteurised milk, soft cheeses made from raw milk, mayonnaise, unpasteurised apple cider, and contaminated vegetables.
- Eating food prepared by people with poor hand hygiene
- Person to person spread.
- Swallowing lake water during swimming,
- Changing diapers,
- Direct contact with farm animals/animal excreta from the petting zoo.
The main reservoir of STEC is the ruminants – cattle, goats, sheep, deer, elk, etc.
Treatment
Treatment is usually supportive. Antibiotics use may increase the risk of complications like HUS; hence it is generally avoided. Anti-motility drugs are also not indicated. In severe cases, antibiotics can be used with specialist guidance. During the O104:H4 outbreak in Germany, many severe cases were treated with azithromycin. [3]
Diagnosis:
Acute bloody diarrhoea can be caused by
- Campylobacter species,
- Shigella species,
- STEC
- Salmonella species,
- Entamoeba histolytica
In the UK, the primary set for faeces for community-acquired gastrointestinal infection usually consists of
- Salmonella,
- Shigella,
- Campylobacter,
- STEC (including O157)
- norovirus
- Giardia species and Cryptosporidium
(There could be local variations) [2]
Lab safety
Faecal specimen processing is performed in a microbiological safety cabinet under containment level 3 conditions as it may contain Hazard Group 3 organisms (S. Typhi, S. Paratyphi A, B, and C, STEC including O157 or Shigella dysenteriae). Laboratory-acquired cases have been reported. [1]

Bacteria
E coli O157 is a motile gram-negative rod (E coli O157:H7; H= flagellar antigen), but non-motile strains are known. They are oxidase negative and usually β-glucuronidase negative (β-glucuronidase positive strains have been reported). They are resistant to tellurite compared to other E. coli.
Non-O157 strains have variable properties.
Laboratories may test the specimen by culture/EIA or molecular methods.
If using culture-based methods in the local laboratory:
Culture plates:
- Cefixime tellurite sorbitol MacConkey (CT-SMAC) agar (incubate on air @ 37 degrees C, 24 hrs).
- STEC chromogenic agar (https://www.chromagar.com/en/product/chromagar-stec/)

– When identifying E coli, some commercial systems may give a doubtful or low percentage profile as sorbitol fermentation is heavily weighted on E coli identification.
– Some E coli O157 could be sorbitol fermenters.
– Escherichia hermannii is sorbitol-negative, cross-reacts and agglutinates in E. coli serotype O157 antiserum; thus, it could be mistaken for E. coli O157.
– MALDI-ToF may not reliably distinguish pathogenic from non-pathogenic E coli. It may also struggle to differentiate E coli from Shigella due to their close genetic relatedness [2]

If using a molecular method in the local laboratory:
- Most use a gastric multiplex PCR – e.g. BD MAX, Serosep etc
- If it is known that a STEC is responsible for the outbreak, specific STEC PCR could be used.
- If there is a strong clinical suspicion of a pathogen targeted by the GI multiplex PCR screening, but the assay is negative, consider additional testing, e.g. enrichment culture with PCR for STEC. Enrichment of STEC – modified tryptone soya broth (MTSB).
- Culture the PCR-positive sample for E coli O157 and send it to the reference lab (as discussed above) for confirmation.
Any Local stx PCR-positive but culture-negative samples should be sent to the Gastrointestinal Bacteria Reference Unit (GBRU), especially with bloody diarrhoea or HUS, for further investigation as various non-O157 STEC serogroups may be identified. [1,2]
Reference laboratory
- Gastrointestinal Bacteria Reference Unit (GBRU) of the Bacteriology Reference Department (BRD) at the Public Health England, Colindale, London.
- The Scottish E. coli Reference Laboratory (SERL) at Edinburgh Royal Infirmary in Scotland.
What to send to the reference laboratory (GBRU):
| CONDITION | CONDITION | SEND |
|---|---|---|
| If it is a case of HUS | the local lab uses a culture-based method | send the faeces sample on the day of receipt |
| If it is a case of HUS | refer to all positive faecal specimens | send the isolate for confirmation, Shiga toxin gene detection and serotyping. |
| If it is a case of HUS | culture / PCR results from GBRU are negative | Consider sending a serum specimen for the detection of antibodies |
| Not a case of HUS | Presumptive E. coli O157 | Send the isolate for identification and Shiga toxin gene detection |
| Not a case of HUS | Local laboratory PCR +ve but culture-negative | Send faeces sample |
| Not a case of HUS, but high suspicion of STEC | Other strains of E coli | Send the isolate for identification and shiga toxin gene detection |
| Not a case of HUS, but bloody diarrhoea | conventional laboratory testing has failed to yield presumptive E. coli O157 or any other pathogen | Send faeces sample |
| Symptomatic contacts of cases of STEC infection or any STEC outbreak-associated case | conventional culture laboratory testing has failed to yield a pathogen | Faeces sample after discussion with GBRU |
Ref [3]
The reference lab offers these tests:
- Test faecal sample for STEC
- PCR – stx1, stx2 (Stx2a, stx2b, stx2c, stx2d etc), eae
- Whole-genome sequencing
- Phage and other typing methods
- serology
Typing methods
Various typing methods are used to help in an outbreak situation. These methods are [1] –
- Phage typing
- Pulsed Field Gel Electrophoresis (PFGE),
- Multilocus Sequence Typing (MLST),
- Multiple-Locus Variable Number Tandem Repeat Analysis (MVLA) and
- Whole Genome Sequencing (WGS).
Infection control
Isolation, strict hand hygiene, and contact precautions.
Patients may be excluded from work, school, or other institutional settings until 48 hours after they have stopped vomiting or having diarrhoea unless they are –
- Any person who cannot perform adequate personal hygiene due to their lack of capacity or ability to comply or lack of access to hygiene facilities,
- All children aged five years old or under (up to their sixth birthday) who attend school, pre-school, nursery, or other similar child care or minding groups
- People whose work involves preparing or serving unwrapped, ready-to-eat food (including drinks)
- Clinical, social care, or nursery staff who work with young children, the elderly, or any other particularly vulnerable people and whose activities increase the risk of transferring infection via the faecal-oral route
If they are symptomatic, wait for a microbiological clearance once the person is symptom-free for ≥48 hours.If they are asymptomatic or recovered, request microbiological clearance once the person is symptom-free for ≥48 hours and risk assess to determine whether restriction/redeployment or a supervised return to childcare settings may be appropriate whilst awaiting results of microbiological testing. If not appropriate, exclude until the microbiological clearance is completed.
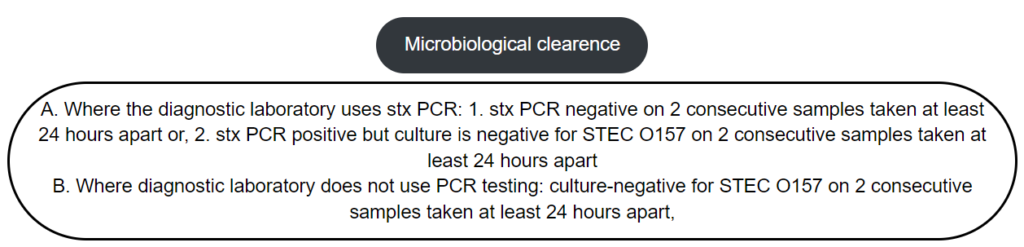
The median duration of shedding bacteria in stool is about 30-40 days, but the range is broad. the duration has an inverse relationship with age. A small number of individuals have been reported to shed the bacteria for up to 6 months. Cases remain infectious until they have ‘cleared’ the infection, i.e. until STEC can no longer be detected in the faeces.
Notification/ referral
- The laboratory should inform the consultant microbiologist as soon as possible.
- The infection control team must be notified of any case in the hospital.
- The diagnostic lab has to notify the UKHSA health protection team, according to the Health Protection (Notification) Regulations (2010), of any presumptive E col O157, within 24 hours and followed up by written notification within seven days. They should notify – Presumptive (locally confirmed) isolates or/& detection of stx DNA from faeces via PCR methods. Consultant microbiologists usually notify the public health team as early as possible by telephone.
- Clinical notification should be made the same day, including out-of-hours, via telephoning the appropriate HPT.
- STEC is on enhanced surveillance – STEC enhanced surveillance questionnaire (to obtain a detailed history for the 7 days before the onset of illness) should be completed (by the health protection team) within 24 hrs of the notification of a probable/confirmed case to the HPT and sent to national Gastrointestinal teams. This is done by the health protection team. [3]
Reference
- https://www.gov.uk/government/publications/smi-id-22-identification-of-escherichia-coli-o157
- https://www.gov.uk/government/publications/smi-s-7-gastroenteritis-and-diarrhoea
- https://www.gov.uk/government/publications/shiga-toxin-producing-escherichia-coli-public-health-management
- https://www.hps.scot.nhs.uk/web-resources-container/guidance-for-the-public-health-management-of-escherichia-coli-o157-and-other-shiga-toxin-producing-stec-infections/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6352002/
Additional note:
Under the Health Protection (Notification) Regulations (2010), diagnostic laboratories notify PHE of the identification of specified causative agents in a human sample (from live or deceased patients) when there is evidence of an infection caused by such an agent. The notifiable causative agents are listed in Schedule 2 of the Regulations (and replicated in Appendix 1). The laboratory uploads the information to The Second Generation Surveillance System (SGSS) from the LIMS using sFTP, web upload or encrypted email.

The notification is done within 7 days, but daily reporting is preferred to enable timely public health action. Local Health Protection Teams (HPTs) should be notified of urgent cases as soon as
reasonably practicable after identifying the causative agent. This should be done orally, usually by telephone. It is recommended that this should always be done within 24 hours. Urgent oral notification should be followed up by written notification within 7 days of identification of the causative agent.


