Management of needlestick injury
This is a study note on managing needlestick/sharps injury or body-fluid exposure. This is not a clinical guideline and must not be used for clinical use. Please discuss this with your doctor, who will be able to advise you based on the most relevant local/regional or national guidelines.
First aid
Needlestick or Sharps injury
– Gently squeeze the wound (DO NOT SUCK AREA)
– Wash the area with soap and warm water (DO NOT SCRUB)
– Cover broken skin with a waterproof dressing
Splash
– Exposed mucous membranes (i.e. mouth/nose), including eyes should be irrigated copiously with water (DO NOT SWALLOW WATER), before and after removing any contact lenses.
The risk of transmission of BBV to an HCW following a needle stick injury is as follows:
- 1 in 3 for Hepatitis B when the Source Patient has Hepatitis B and is e Ag positive
- 1 in 30 for Hepatitis C when the Source Patient has Hepatitis C
- 1 in 300 for HIV when the Source Patient has HIV
Q1. Is it a significant exposure?
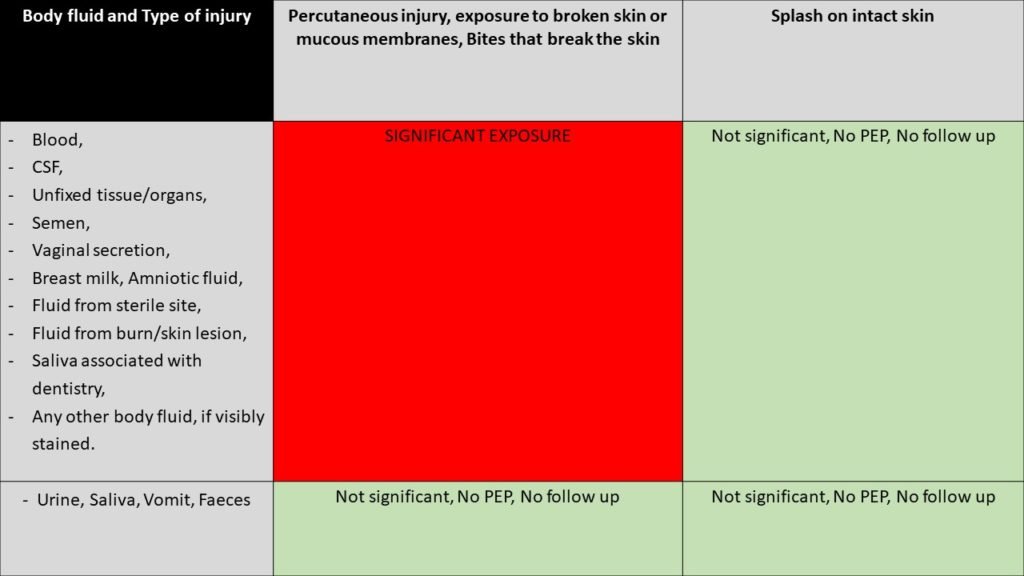
If NO - assure, but check the hepatitis B status. See the chart below.
If Yes - Obtain blood from the recipient and store the blood (it is usually stored for two years).
Q2. Assess the needlestick donor (source patient)
Take consent from the donor:
Consent donor/source for Hep B, Hep C & HIV testing. Consent MUST be obtained for the test and disclosing the result to the recipient. Consent should be taken by a healthcare professional other than the recipient.
Q2a. Which test to request on donor blood?
| BBV | Which test to request? | Further action if positive (for source/donor) |
|---|---|---|
| Hepatitis B | Hepatitis B surface antigen | If positive, work up by testing other hepatitis markers and HBV PCR. The patient may need a hepatologist/ Infectious diseases referral. |
| Hepatitis C | Hepatitis C antibody/antigen | If HCV ab positive - test HCV RNA (Hepatitis C PCR). Immunocompromised patients or patients with features suggestive of acute Hepatitis should be tested for HCV PCR even if the HCV antibody is negative. |
| HIV | HIV antibody/Antigen | Confirmation, viral load and appropriate referral for management. |
Q2b. What if the donor is unconscious?
- Where a source patient lacks the capacity to consent (e.g. because they are unconscious), his/her tissue etc can only lawfully be tested for serious communicable diseases if it is reasonably held to be in his/her best interests in accordance with the Mental Capacity Act 2005.
- The General Medical Council, therefore, withdrew its guidance that set out exceptional circumstances in which the testing of an unconscious patient may be undertaken without their consent.
Q2c. What if the donor is deceased?
1. If a patient specifically refused testing prior to death, then the wish must be upheld post-mortem.
2. If the patient prior to his/her death, has nominated an individual to provide consent for medical interventions, then this individual can provide consent for testing.
3. A person in a "qualifying relationship" can give consent. The Human Tissue Authority’s Code of Practice (2004) states: “Consent is needed from only one person in the hierarchy of qualifying relationships and should be obtained from the person ranked highest. If a person high up the list refuses to give consent, it is not possible to act on consent from someone further down the list.”
- Spouse, civil partner or partner,
- Parent or child,
- Brother or sister,
- Grandparent or grandchild,
- Child of a person falling within paragraph (c)
- Stepfather or stepmother
- Half-brother or half-sister
- Friend of longstanding.
4. If none of the above is applicable or available to provide consent, then consent should be sort from the Coroner.
Q2d. Source unidentifiable/unavailable (e.g. discarded needle)
Manage as unknown source exposure (see below) – it is seldom appropriate to test discarded needles and syringes; they should generally be safely disposed of instead.
Q4. What post-exposure prophylaxis (PEP) to give?
Hepatitis B

Please note Children born in the UK should have 3 doses of the Hepatitis B vaccine by 16 weeks. It was incorporated into the routine immunisation schedule in late 2017. When assessing for hep B, take this information into consideration.
HIV
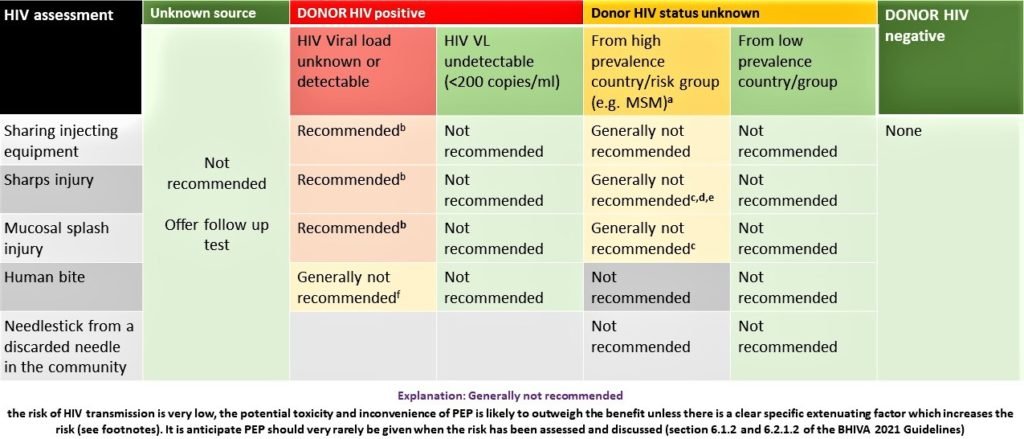

HIV Post-Exposure Prophylaxis after needlestick injury
- If eligible, give PEP preferably within an hour of exposure, and certainly no later than 48-72 hours.
- Discuss with the recipient and take into account their view regarding PEP and take their consent.
- All individuals commencing PEP should have a test (rather than just a specimen for storage) for HIV as in the unlikely event that they have HIV, treatment with 28 days of PEP alone would result in potential harm as resistance to the treatments given could arise once this is stopped at the end of the course.
Regimen
- One Truvada tablet (245mg tenofovir and 200mg emtricitabine) once a day
- plus
- One Raltegravir tablet (400mg Raltegravir) twice a day
- for 4 weeks
(or check local guidelines)
There is no PEP for Hepatitis C
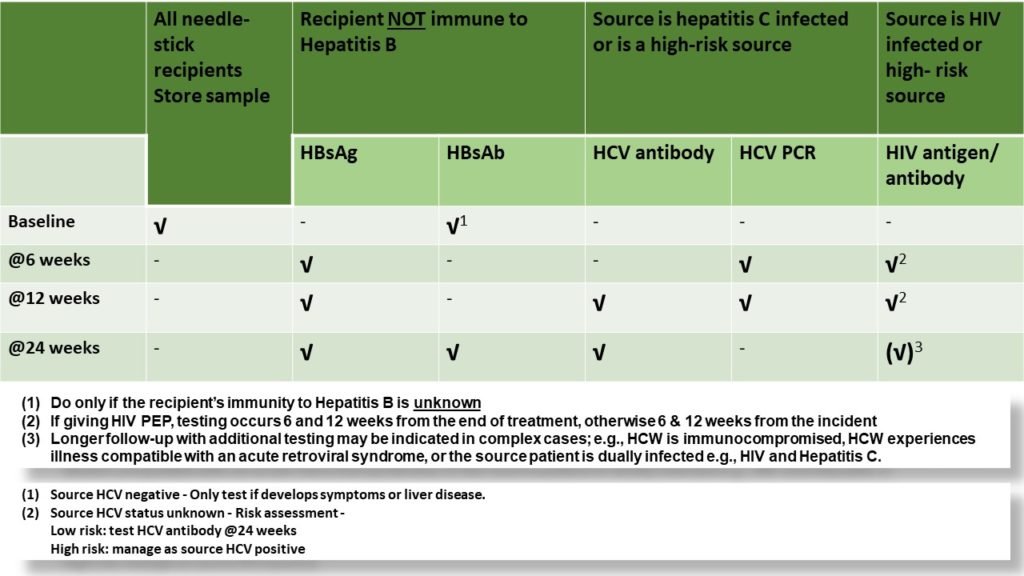
What are the blood tests required after a needlestick injury or follow-up tests for the recipient?
Reporting
- Any sharps injury to the staff should be reported to occupational health.
- Incident form/Datix.
- Occupational health (on behalf of the trust), under RIDDOR, should report to Health and Safety Executives within 10 days.
- Any occupationally acquired HepB, HepC, or HIV must be reported to the local health protection unit in confidence by occupational health.
- Any HepB, HepC, or HIV that the patient may have acquired from a BBV-infected staff due to a sharps injury must be reported to the local health protection unit in confidence by an infection control doctor/microbiologist.

