Dengue vaccine - Greenbook Summary
Vaccine Description and Composition
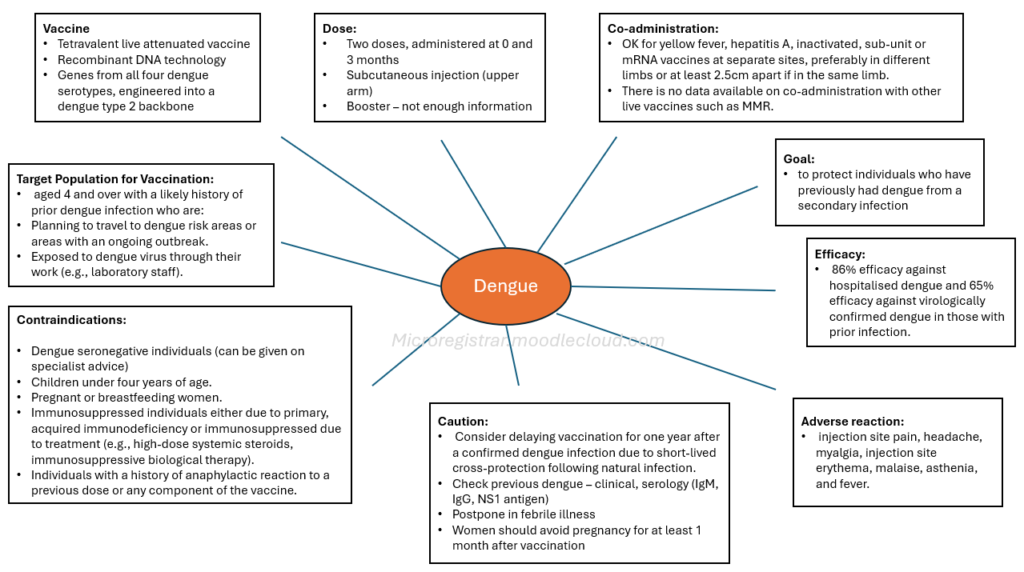
- Type: Qdenga® is a tetravalent live attenuated vaccine, meaning it uses a weakened form of the virus to stimulate an immune response.
- Production: It is produced using recombinant DNA technology in Vero cells.
- Serotypes: The vaccine contains serotype-specific surface protein genes from all four dengue serotypes, engineered into a dengue type 2 backbone. This means it aims to protect against all four types of the dengue virus.
- Availability: Qdenga® is available in the UK, unlike another dengue vaccine, Dengvaxia, which is not available in the UK and should not be interchanged.
"The dengue vaccination Qdenga® is a tetravalent live attenuated vaccine. It is produced in Vero cells by recombinant DNA technology and contains serotype-specific surface protein genes of the four dengue serotypes, engineered into a dengue type 2 backbone."
Storage and Presentation
- Storage: Qdenga® must be stored in its original packaging at +2°C to +8°C, protected from light. Temperature control is critical to maintain the vaccine's potency. Freezing should be avoided.
"Vaccines should be stored in the original packaging at +2C̊ to +8C̊ and protected from light... Heat speeds up the decline in potency of most vaccines... Freezing may cause increased reactogenicity and loss of potency"
- Presentation: The vaccine is presented as a freeze-dried powder (a "compact cake") that requires reconstitution with a clear, colourless solvent before use.
Dosage and Administration
- Dosage: The standard dose is 0.5 mL.
- Schedule: The vaccination schedule consists of two doses, administered at 0 and 3 months. The need for a booster dose is not currently established.
- Route of Administration: The vaccine should be administered via subcutaneous injection, preferably in the upper arm in the region of the deltoid.
- Co-administration: Qdenga® can be co-administered with yellow fever, hepatitis A, inactivated, sub-unit or mRNA vaccines at separate sites, preferably in different limbs or at least 2.5cm apart if in the same limb. There is no data available on co-administration with other live vaccines such as MMR.
Recommendations for Use
- Primary Objective: The primary objective of the UK immunisation program is to protect individuals who have previously had dengue from a secondary infection, which carries a greater risk of severity.
- Efficacy: In trials, the vaccine was found to have 86% efficacy against hospitalised dengue and 65% efficacy against virologically confirmed dengue in those with prior infection.
- Not Recommended for Seronegative Individuals: The vaccine is not recommended for individuals with no prior history of dengue infection ("seronegative") due to a theoretical risk of severe dengue if these individuals are subsequently exposed to DENV3 or DENV4.
- Target Population for Vaccination: Vaccination is considered for individuals aged 4 and over with a likely history of prior dengue infection who are:
- Planning to travel to dengue risk areas or areas with an ongoing outbreak.
- Exposed to dengue virus through their work (e.g., laboratory staff).
- Delay After Infection: Consider delaying vaccination for one year after a confirmed dengue infection due to short-lived cross-protection following natural infection.
- Determining Previous Infection
- Importance: Decisions about vaccination rely on obtaining a reliable history of dengue infection, considering previous travel, illness, vaccination history, and laboratory results.
- Laboratory Confirmation: A previous dengue infection is reliably confirmed by a positive PCR test at the time of illness, detecting viral RNA.
- Dengue serology (IgM and IgG) results are more complex to interpret due to cross-reactivity with other flaviviruses and vaccinations. A positive IgG alone requires a careful assessment of possible causes, including other flavivirus exposure/vaccination and travel history. A positive NS1 antigen test should be confirmed by serology 4 weeks later
- Clinical History: In the absence of reliable laboratory confirmation, factors such as residence in an endemic area and a history of compatible illness (fever of 2-7 days with 2 or more of: headache, retro-orbital pain, myalgia, arthralgia, rash, thrombocytopenia, leucopenia) can be considered when deciding whether to vaccinate, along with the likelihood of prior exposure.
- Uncertainty: Where uncertainty exists, the potential risks of vaccination should be clearly explained.
- Referral Cases where PCR test results are positive should be discussed with the Rare and Imported Pathogens Laboratory (RIPL)
Contraindications
Qdenga® should not be given to:
- Children under four years of age.
- Pregnant or breastfeeding women.
- Individuals with primary or acquired immunodeficiency (including symptomatic or impaired immune function with asymptomatic HIV).
- Individuals who are immunosuppressed due to treatment (e.g., high-dose systemic steroids, immunosuppressive biological therapy).
- Individuals with a history of anaphylactic reaction to a previous dose or any component of the vaccine.
Precautions
- Vaccination should be postponed in individuals with an acute severe febrile illness.
- Women of childbearing potential should avoid pregnancy for at least one month after vaccination.
- Consider delaying administration of a Qdenga® vaccination for a period of one year after a laboratory-confirmed infection.
- In exceptional circumstances, vaccination can be considered for seronegative individuals, but specialist advice should be sought.
Adverse Reactions
- Common Reactions: The most commonly reported reactions include injection site pain, headache, myalgia, injection site erythema, malaise, asthenia, and fever.
- Duration: These reactions are typically mild to moderate in severity, occur within 2 days of vaccination, and last 1-3 days.
- Frequency: Adverse reactions are often less frequent after the second injection.
- Monitoring As a newly licensed product, the vaccine is under enhanced surveillance ('black triangle' status) and all suspected adverse drug reactions (ADRs) should be reported via the yellow card scheme and to the manufacturers, Takeda UK Ltd.
Management of Cases
- Severe Dengue: Acute samples from suspected cases of severe dengue should be sent to the RIPL for investigation. Previous dengue vaccination history should be obtained.
- Reporting Healthcare professionals should liaise with their regional Infectious Disease team and report cases of suspected severe dengue following vaccination in line with serious adverse incident guidance.
- Treatment: No specific therapy for dengue is available. NSAIDs such as aspirin and ibuprofen should be avoided, as they increase bleeding risk. Severe dengue requires prompt recognition and hospitalisation for supportive treatment.